
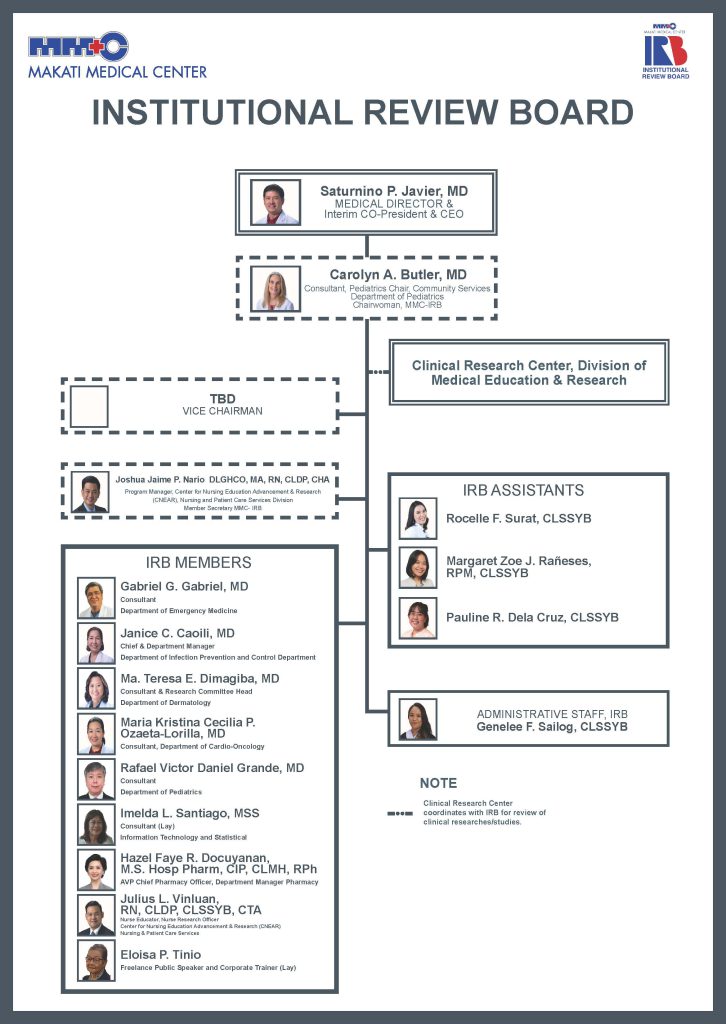
Makati Medical Center Institutional Review Board (MMC IRB): Composition and Functions
The Makati Medical Center Institutional Review Board (MMC IRB) plays a critical role in ensuring that all research conducted within the institution adheres to the highest ethical standards and complies with relevant regulatory requirements. The IRB’s structure reflects a commitment to protecting research participants while supporting the integrity and quality of scientific investigations.
1. Multidisciplinary Composition
The IRB is composed of professionals from a wide range of disciplines relevant to health research. This multidisciplinary makeup ensures that protocol reviews are conducted from multiple perspectives, incorporating clinical, ethical, technical, and community-based viewpoints.
2. Membership Breakdown
- Five Physicians, each representing a distinct medical specialty:
- Pediatrics
- Infectious Diseases
- Emergency Medicine
- Dermatology
- Cardiology-Oncology
Their diverse clinical expertise ensures that studies are evaluated with a deep understanding of specific patient populations and medical interventions.
- Two Nurses
Provide essential insights into patient care, safety, and advocacy, especially from the nursing and bedside perspective. - One Pharmacist
Brings critical expertise in drug-related research, including medication safety, dosage, and pharmaceutical ethics. - Two Non-Institutional (Lay) Members:
- Information Technology and Statistical Consultant: Offers expertise in data management, analysis, and digital systems used in research.
- Human Resource Development and Training Expert: Provides a perspective on organizational practices, participant training, and research team competence.
3. IRB Assistant and Administrative Staff
Support personnel play an essential role in the board’s day-to-day operations:
- Ensure completeness and accuracy of all submissions from principal investigators.
- Monitor both pre- and post-approval documents and processes.
- Guide researchers in adherence to Good Clinical Practice (GCP).
- Oversee both administrative and operational functions to maintain efficiency and compliance.
4. Qualifications and Experiences
All IRB members possess qualifications and professional experience relevant to their disciplines, equipping them to assess both the scientific rigor and ethical dimensions of research protocols.
5. Expertise Pool
The IRB maintains a panel of GCP-certified independent consultant reviewers who are engaged when studies require specialized knowledge not covered by the regular board. This ensures that complex or niche protocols receive expert evaluation when necessary.
6. Conclusion
The MMC IRB’s structure and processes reflect a deep commitment to ethical research conduct. Through its multidisciplinary board, experienced staff, and access to specialized consultants, the IRB upholds the rights and safety of research participants and ensures the scientific integrity of all research conducted within Makati Medical Center.
Makati Medical Center IRB Table of Organization
FERCAP Recognition and Certificate of Accreditation


